How To Draw Constant Volume Lines On A Ts Diagram
Thermodynamics is a branch of physics which deals with the energy and work of a system. Thermodynamics deals with the large scale response of a system which we can observe and measure in experiments. As aerodynamicists, nosotros are most interested in the thermodynamics of propulsion systems and high speed flows. The propulsion system of an shipping generates thrust by accelerating a working fluid, usually a heated gas. In response to Newton's tertiary law of motion, the propulsion organisation and the aircraft are accelerated in the opposite management. To understand how a propulsion arrangement works, nosotros must study the basic thermodynamics of the working fluid.
The working fluid of a propulsion arrangement is a gas. Gases have various properties that we can observe with our senses, including the gas pressure level p, temperature T, mass, and volume V that contains the gas. Conscientious, scientific ascertainment has adamant that these variables are related to ane some other, and the values of these properties decide the state of the gas. The first and second laws of thermodynamics define two additional variables, enthalpy and entropy S, which can also exist used to draw the state of a gas. A thermodynamic procedure, such as heating or compressing the gas, changes the values of the state variables in a prescribed manner. The full work and estrus transferred to a gas depend on the beginning and ending states of the gas and on the process used to change the state.
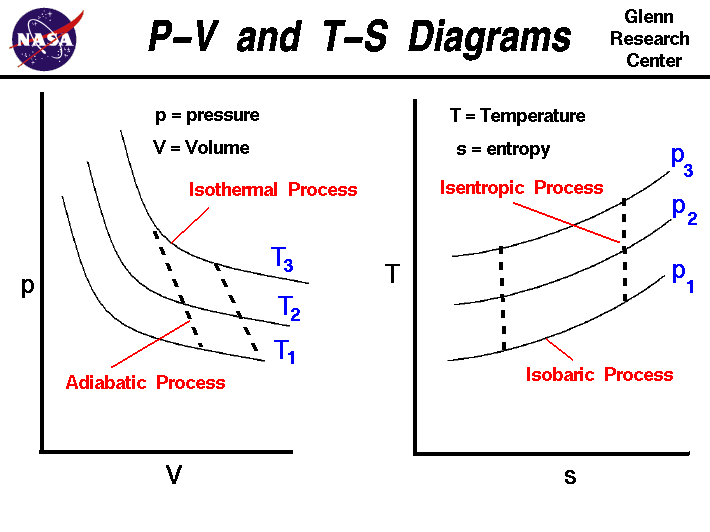
It is useful to plot the changes in the land of a gas during a thermodynamic process. On the figure we show two types of plots that are used to draw changes of country. On the left we have plotted the pressure versus the volume, which is called a p-V diagram. On a p-Five diagram, lines of constant temperature curve from the upper left to the lower right. A process performed at constant temperature is called an isothermal process. During an adiabatic process no heat is transferred to the gas, simply the temperature, pressure level, and volume of the gas change as shown by the dashed line. As described on the work slide, the expanse under a process curve on a p-V diagram is equal to the work performed by a gas during the process. On the right of the effigy we accept plotted the temperature versus the entropy of the gas. This plot is called a T-s diagram. Lines of constant pressure curve from the lower left to upper right on a T-s diagram. A constant pressure level process is called an isobaric process and this type of procedure occurs in the combustor of a gas turbine engine. During an isentropic procedure in that location is no alter in the entropy of the system and the process is reversible. An isentropic process appears equally a vertical line on a T-s diagram. The area under a procedure bend on a T-south diagram is related to the amount of heat transferred to the gas.
It is possible to perform a series of processes, in which the land is changed during each process, but the gas eventually returns to its original country. Such a series of processes is chosen a cycle and forms the basis for understanding engines. The Carnot Cycle describes the operation of refrigerators, the Otto Cycle describes the operation of internal combustion engines, and the Brayton Cycle describes the operation of gas turbine engines. P-V and T-due south diagrams are ofttimes used to visualize the processes in a thermodynamic bike and assist us amend sympathise the thermodynamics of engines.
Activities:
Guided Tours
Navigation..
- Beginner'southward Guide Home Page
Source: https://www.grc.nasa.gov/www/k-12/airplane/pvtsplot.html
Posted by: davisbantais.blogspot.com


0 Response to "How To Draw Constant Volume Lines On A Ts Diagram"
Post a Comment